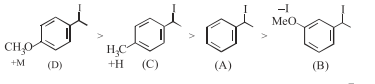
Question: Increasing rate of $S_{N} 1$ reaction in the following compounds is :
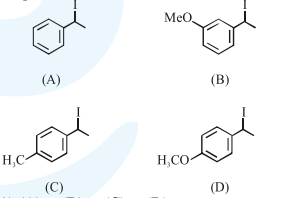
$(\mathrm{A})<(\mathrm{B})<(\mathrm{C})<(\mathrm{D})$
$(\mathrm{B})<(\mathrm{A})<(\mathrm{D})<(\mathrm{C})$
$(\mathrm{B})<(\mathrm{A})<(\mathrm{C})<(\mathrm{D})$
$(\mathrm{A})<(\mathrm{B})<(\mathrm{D})<(\mathrm{C})$
Correct Option: , 3
Solution:
Rate of $\mathrm{S}_{\mathrm{N}^{*}}$ is directly proposional to stability of first formed carbocation so answer is