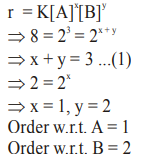Question:
For the reaction, $2 \mathrm{~A}+\mathrm{B} \rightarrow$ products, when the concentrations of A and B both wrere doubled, the rate of the reaction increased from $0.3 \mathrm{~mol}$ $\mathrm{L}^{-1} \mathrm{~S}^{-1}$ to $2.4 \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}$. When the concentration of $\mathrm{A}$ alone is doubled, the rate increased from $0.3 \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}$ to0.6 $\mathrm{mol} \mathrm{L}^{-1} \mathrm{~s}^{-1}$
Which one of the following statements is correct?
Correct Option: 1
Solution: