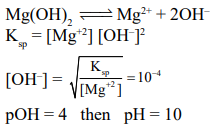Question:
At $25^{\circ} \mathrm{C}$, the solubility producct of $\mathrm{Mg}(\mathrm{OH})_{2}$ is $1.0 \times 10^{-11}$. At which pH, will $\mathrm{Mg}^{2+}$ ions start precipitating in the form of $\mathrm{Mg}(\mathrm{OH})_{2}$ from a solution of $0.001 \mathrm{M} \mathrm{Mg}^{2+}$ ions ?
Correct Option: , 3
Solution: