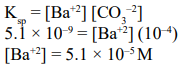Question:
Solid Ba(NO $)_{3}$ is gradully dissolved in a $1.0 \times 10^{-4} \mathrm{M} \mathrm{Na}_{2} \mathrm{CO}_{3}$ solution. At what concentration of $\mathrm{Ba}^{2+}$ will a precipitate begin to form?
$\left(\mathrm{K}_{\mathrm{SP}}\right.$ for $\left.\mathrm{Ba} \mathrm{CO}_{3}=5.1 \times 10^{-9}\right)$
Correct Option: , 4
Solution: