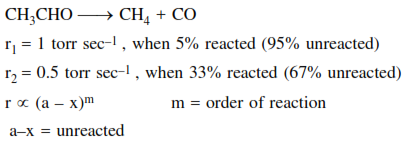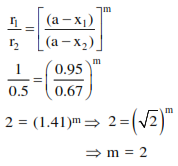Question:
At $518^{\circ} \mathrm{C}$, the rate of decomposition of a sample of gaseous acetaldehyde, initially at a pressure of 363 Torr, was $1.00$ Torr s $^{-1}$ when $5 \%$ had reacted and $0.5$ Torr $^{-1}$ when $33 \%$ had reacted. The order of the reaction is:
Correct Option: , 4
Solution: