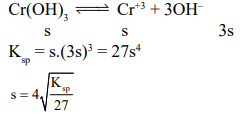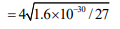Question: The $\mathrm{K}_{\mathrm{sp}}$ for $\mathrm{Cr}(\mathrm{OH})_{3}$ is $1.6 \times 10^{-30}$. The molar solubility of this compound in water is :-
$\sqrt[2]{1.6 \times 10^{-30}}$
$\sqrt[4]{1.6 \times 10^{-30}}$
$\sqrt[4]{1.6 \times 10^{-30} / 27}$
$1.6 \times 10^{-30} / 27$
Correct Option: , 3
Solution: