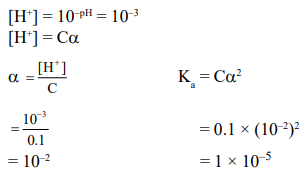Question: The $\mathrm{pH}$ of a $0.1$ molar solution of the acid $\mathrm{HQ}$ is 3 . The value of the ionization constant, $\mathrm{K}_{\mathrm{a}}$ of this acid is :-
$1 \times 10^{-7}$
$3 \times 10^{-7}$
$1 \times 10^{-3}$
$1 \times 10^{-5}$
Correct Option: , 4
Solution: