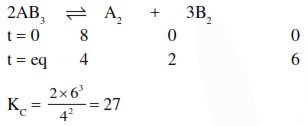Question:
$8 \mathrm{~mol}$ of $\mathrm{AB}_{3}(\mathrm{~g})$ are introduced into a $1.0 \mathrm{dm}^{3}$ vessel. If it dissociates as $2 \mathrm{AB}_{3}(\mathrm{~g}) \rightleftharpoons \mathrm{A}_{2}(\mathrm{~g})+3 \mathrm{~B}_{2}(\mathrm{~g})$ At equilibrium, $2 \mathrm{~mol}$ of $\mathrm{A}_{2}$ are found to be present. The equilibrium constant of this reaction is :-
Correct Option: , 3
Solution: