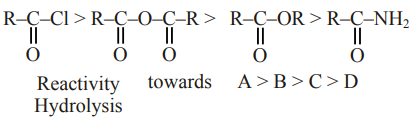
Question: 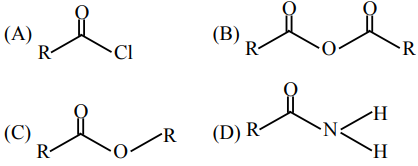
The correct order of their reactivity towards hydrolysis at room temperature is :
$(\mathrm{A})>(\mathrm{B})>(\mathrm{C})>(\mathrm{D})$
$(\mathrm{D})>(\mathrm{A})>(\mathrm{B})>(\mathrm{C})$
$(\mathrm{D})>(\mathrm{B})>(\mathrm{A})>(\mathrm{C})$
$(\mathrm{A})>(\mathrm{C})>(\mathrm{B})>(\mathrm{D})$
Correct Option: 1,
Solution: