Question:
$100 \mathrm{~mL}$ of $\mathrm{Na}_{3} \mathrm{PO}_{4}$ solution contains $3.45 \mathrm{~g}$ of sodium. The molarity of the solution is $\times 10^{-2}$ $\mathrm{mol} \mathrm{L}^{-1}$. (Nearest integer)
[Atomic Masses - $\mathrm{Na}: 23.0 \mathrm{u}, \mathrm{O}: 16.0 \mathrm{u}, \mathrm{P}: 31.0 \mathrm{u}$ ]
Solution:
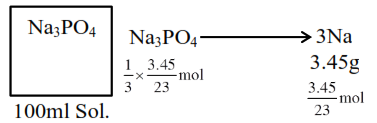
therefore molarity of $\mathrm{Na}_{3} \mathrm{PO}_{4}$ Solution $=$ $\frac{\mathrm{n}_{\mathrm{Na}_{3} \mathrm{PO}_{4}}}{\text { volume of solution in } \mathrm{L}}$
$=\frac{\frac{1}{3} \times \frac{3.45}{23} \mathrm{~mol}}{0.1 \mathrm{~L}}$
$=0.5=50 \times 10^{-2}$
