Question:
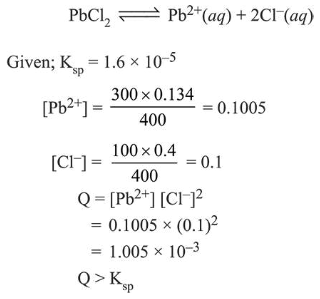
The $\mathrm{K}_{\mathrm{sp}}$ for the following dissociation is $1.6 \times 10^{-5}$
$\mathrm{PbCl}_{2(\mathrm{~s})} \rightleftharpoons \mathrm{Pb}_{(a q)}^{2+}+2 \mathrm{Cl}_{(a q)}^{-}$
Which of the following choices is correct for a mixture of $300 \mathrm{~mL} 0.134 \mathrm{M} \mathrm{Pb}\left(\mathrm{NO}_{3}\right)_{2}$ and $100 \mathrm{~mL} 0.4 \mathrm{M} \mathrm{NaCl}$ ?
Correct Option: , 3
Solution: