Question:
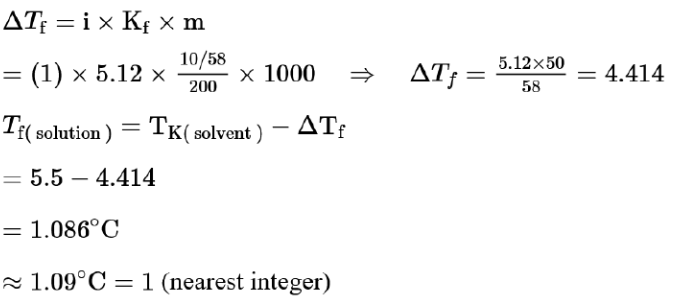
$\mathrm{C}_{6} \mathrm{H}_{6}$ freezes at $5.5^{\circ} \mathrm{C}$. The temperature at which a solution of $10 \mathrm{~g}$ of $\mathrm{C}_{4} \mathrm{H}_{10}$ in $200 \mathrm{~g}$ of $\mathrm{C}_{6} \mathrm{H}_{6}$ freeze is__________ ${ }^{\circ} \mathrm{C}$. (The molal freezing point depression constant of $\mathrm{C}_{6} \mathrm{H}_{6}$ is) $5.12^{\circ} \mathrm{C} / \mathrm{m}$ )
Solution:
(1)