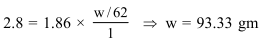Question:
$\mathrm{K}_{\mathrm{f}}$ for water is $1.86 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}{ }^{-1}$. If your automobile radiator holds $1.0 \mathrm{~kg}$ of water, how many grams of ethylene glycol $\left(\mathrm{C}_{2} \mathrm{H}_{6} \mathrm{O}_{2}\right)$ must you add to get the freezing point of the solution lowered to $-2.8^{\circ} \mathrm{C} ?$
Correct Option: , 3
Solution: