Question: The molecular geometry of $\mathrm{SF}_{6}$ is octahedral. What is the geometry of $\mathrm{SF}_{4}$ (including lone pair(s) of electrons, if any)?
Tetrahedral
Trigonal bipyramidal
Pyramidal
Square planar
Correct Option: , 2
Solution:
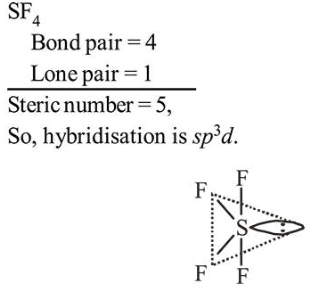
Geometry is trigonal bipyramidal but shape is "See Saw".