$2 \mathrm{NO}(\mathrm{g})+\mathrm{Cl}_{2}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{NOCl}(\mathrm{s})$ This reaction was studied at $-10^{\circ} \mathrm{C}$ and the following data was obtained
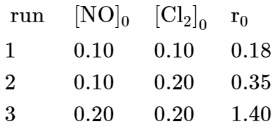
$[\mathrm{NO}]_{0}$ and $\left[\mathrm{Cl}_{2}\right]_{0}$ are the initial concentrations and $\mathrm{r}_{0}$ is the initial reaction rate. The overall order of the reaction is _______________ (Round off to the Nearest Integer).
(3)
$\mathrm{r}=\mathrm{k}[\mathrm{NO}]^{\mathrm{m}}\left[\mathrm{Cl}_{2}\right]^{\mathrm{n}}$
$=\mathrm{k}(0.1)^{\mathrm{m}}(0.1)^{\mathrm{n}}$
$=\mathrm{k}(0.1)^{\mathrm{m}}(0.2)^{\mathrm{n}} \quad \ldots .(1)$
$=\mathrm{k}(0.2)^{\mathrm{m}}(0.2)^{\mathrm{n}} \quad \ldots(3)$
$\mathrm{n}=1$
$\mathrm{~m}=2$
$\mathrm{m}+\mathrm{n}=3$
