Question:
$2 \mathrm{SO}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g}) \rightarrow 2 \mathrm{SO}_{3}(\mathrm{~g})$
The above reaction is carried out in a vessel starting with partial pressure $\mathrm{P}_{\mathrm{SO}_{2}}=250 \mathrm{~m}$ bar, $\mathrm{P}_{\mathrm{O}_{2}}=750 \mathrm{~m}$ bar and $\mathrm{P}_{\mathrm{SO}_{3}}=0$ bar. When the reaction is complete, the total pressure in the reaction vessel is__________ $\mathrm{m}$ bar. (Round off of the nearest integer).
Solution:
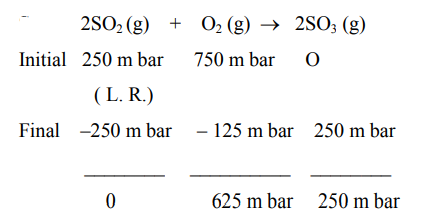
$\therefore$ Final total pressure $=625+250=875 \mathrm{~m}$ bar
