Question:
One mole of $\mathrm{H}_{2} \mathrm{O}$ and one mole of $\mathrm{CO}$ are taken in $10 \mathrm{~L}$ vessel and heated to
725 K. At equilibrium 40% of water (by mass) reacts with CO according to the equation,
$\mathrm{H}_{2} \mathrm{O}(\mathrm{g})+\mathrm{CO}(\mathrm{g}) \longleftrightarrow \mathrm{H}_{2}(\mathrm{~g})+\mathrm{CO}_{2}(\mathrm{~g})$
Calculate the equilibrium constant for the reaction.
Solution:
The given reaction is:
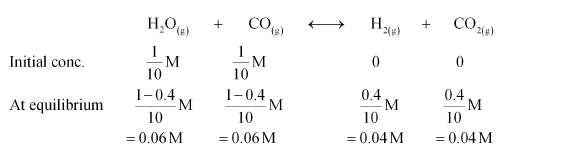
Therefore, the equilibrium constant for the reaction,
$K_{\mathrm{C}}=\frac{\left[\mathrm{H}_{2}\right]\left[\mathrm{CO}_{2}\right]}{\left[\mathrm{H}_{2} \mathrm{O}\right][\mathrm{CO}]}$
$=\frac{0.04 \times 0.04}{0.06 \times 0.06}$
$=0.444$ (approximately)
