Question:
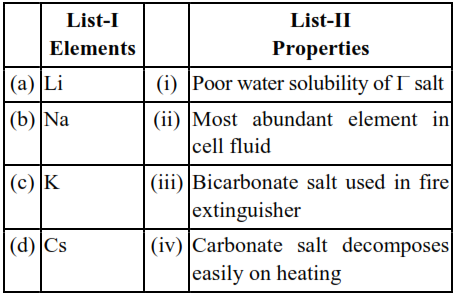
Match List I with List II :
Choose the correct answer from the options given below:
Correct Option: 1
Solution:
(a) $\mathrm{C}_{\mathrm{S}} \mathrm{I}$ salt is poor water soluble due to it's low hydration energy
(b) $\mathrm{NaHCO}_{3}$ is used in fire extinguisher
(c) $\mathrm{K}$ is most abundant element in cell fluid
(d) $\mathrm{Li}_{2} \mathrm{CO}_{3}$ decomposes easily due to high covalent character caused by small size $\mathrm{Li}^{+}$cation.
