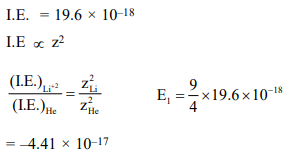Question: Ionisation energy of $\mathrm{He}^{+}$is $19.6 \times 10^{-18} \mathrm{~J}_{\text {atom }^{-1}}$. The energy of the first stationary state $(\mathrm{n}=1)$
$8.82 \times 10^{-17} \mathrm{~J}$ atom $^{-1}$
$4.41 \times 10^{-16} \mathrm{~J}_{\text {atom }}-1$
$-4.41 \times 10^{-17} \mathrm{~J}_{\text {atom }^{-1}}$
$-2.2 \times 10^{-15} \mathrm{~J}$ atom ${ }^{-1}$
Correct Option: , 3
Solution: