Indicate the number of unpaired electrons in: (a) $\mathrm{P}$, (b) $\mathrm{Si}$, (c) $\mathrm{Cr}$, (d) Fe and (e) $\mathrm{Kr}$.
(a) Phosphorus (P):
Atomic number $=15$
The electronic configuration of $P$ is:
$1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{3}$
The orbital picture of $P$ can be represented as:
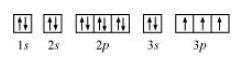
From the orbital picture, phosphorus has three unpaired electrons.
(b) Silicon (Si):
Atomic number = 14
The electronic configuration of Si is:
$1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{2}$
The orbital picture of chromium is:

From the orbital picture, silicon has two unpaired electrons.
(c) Chromium (Cr):
Atomic number $=24$
The electronic configuration of $\mathrm{Cr}$ is:
$1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4 s^{1} 3 d^{5}$
The orbital picture of chromium is:
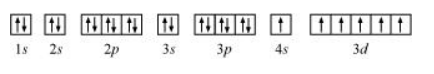
From the orbital picture, chromium has six unpaired electrons.
(d) Iron (Fe):
Atomic number $=26$
The electronic configuration is:
$1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4 s^{2} 3 d^{6}$
The orbital picture of chromium is:
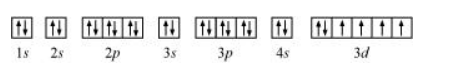
From the orbital picture, iron has four unpaired electrons.
(e) Krypton (Kr):
Atomic number $=36$
The electronic configuration is:
$1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4 s^{2} 3 d^{10} 4 p^{6}$
The orbital picture of krypton is:
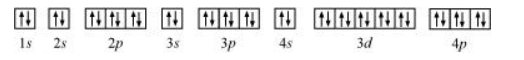
Since all orbitals are fully occupied, there are no unpaired electrons in krypton.
