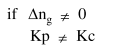Question: In which one of the following equilibria, $\mathrm{K}_{\mathrm{p}} \neq \mathrm{K}_{\mathrm{c}}$ ?
$\mathrm{NO}_{2}(\mathrm{~g})+\mathrm{SO}_{2}(\mathrm{~g}) \rightleftharpoons \mathrm{NO}(\mathrm{g})+\mathrm{SO}_{3}(\mathrm{~g})$
$2 \mathrm{HI}(\mathrm{g}) \rightleftharpoons \mathrm{H}_{2}(\mathrm{~g})+\mathrm{I}_{2}(\mathrm{~g})$
$2 \mathrm{NO}(\mathrm{g}) \rightleftharpoons \mathrm{N}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g})$
$2 \mathrm{C}(\mathrm{s})+\mathrm{O}_{2}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{CO}(\mathrm{g})$
Correct Option: , 4
Solution: