Question:
In which of the following order the given complex ions are arranged correctly with respect to their decreasing spin only magnetic moment ?
$\left[\mathrm{FeF}_{6}\right]^{3-}$
$\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}$
$\left[\mathrm{NiCl}_{4}\right]^{2-}$
$\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}$
Correct Option: 1
Solution:
Complex
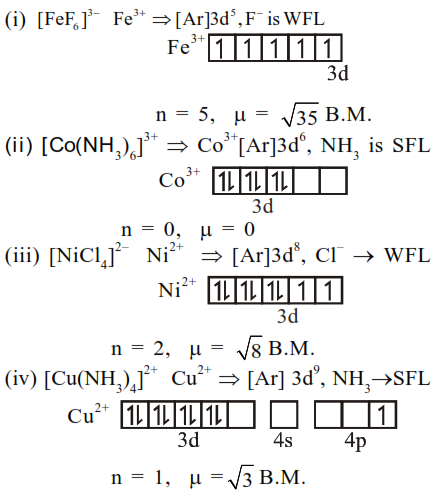
Thus correct order of spin only magnetic moment is (i) $>$ (iii) $>$ (iv) $>$ (ii)
