Question:
In Tollen's test for aldehyde, the overall number of electron(s) transferred to the Tollen's reagent formula $\left[\mathrm{Ag}\left(\mathrm{NH}_{3}\right)_{2}\right]^{+}$per aldehyde group to form silver mirror is . _____________(Round off to the Nearest integer)
Solution:
(2)
$\mathrm{AgNO}_{3}+\mathrm{NaOH} \rightarrow \mathrm{AgOH}+\mathrm{NaNO}_{3}$
$2 \mathrm{AgOH} \rightarrow \mathrm{Ag}_{2} \mathrm{O}+\mathrm{H}_{2} \mathrm{O}$
$\mathrm{Ag}_{2} \mathrm{O}+4 \mathrm{NH}_{3}+\mathrm{H}_{2} \mathrm{O} \rightarrow 2 \mathrm{Ag}\left(\mathrm{NH}_{3}\right)_{2}^{+}+2 \mathrm{OO}$
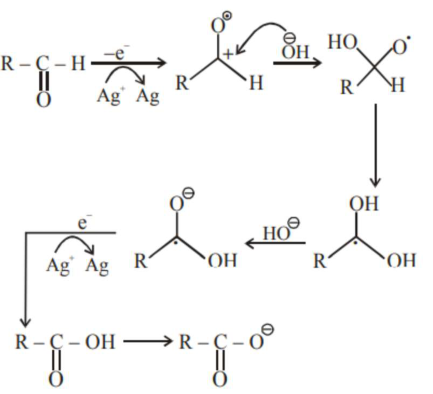
Total $2 \mathrm{e}^{-}$transfer to Tollen's reagent
