Question.
In the Modern Periodic Table, calcium (atomic number 20) is surrounded by elements with atomic numbers 12, 19, 21, and 38. Which of these have physical and chemical properties resembling calcium?
In the Modern Periodic Table, calcium (atomic number 20) is surrounded by elements with atomic numbers 12, 19, 21, and 38. Which of these have physical and chemical properties resembling calcium?
solution:
The elements with atomic number 12 and 38 have same chemical properties as that of calcium. This is because both of them have same number of valence electrons (2) as calcium.
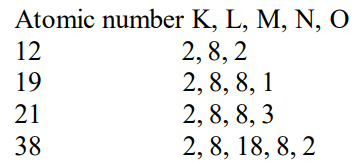
Since elements with same number of valence electron have similar properties, the elements which have 2 valence electrons will have physical and chemical properties resembling that of calcium
The elements with atomic number 12 and 38 have same chemical properties as that of calcium. This is because both of them have same number of valence electrons (2) as calcium.
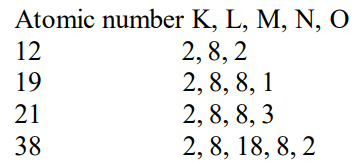
Since elements with same number of valence electron have similar properties, the elements which have 2 valence electrons will have physical and chemical properties resembling that of calcium
