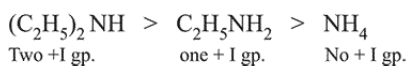Question: In the following compounds, the decreasing order of basic strength will be:
$\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}>\mathrm{NH}_{3}>\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$
$\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}>\mathrm{NH}_{3}>\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}$
$\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}>\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}>\mathrm{NH}_{3}$
$\mathrm{NH}_{3}>\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{NH}_{2}>\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \mathrm{NH}$
Correct Option: , 3
Solution: