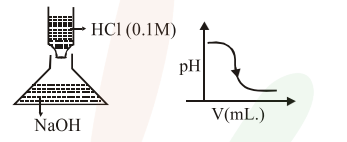
Question:
In an acid-base titration, $0.1 \mathrm{M} \mathrm{HCl}$ solution was added to the $\mathrm{NaOH}$ solution of unknown strength. Which of the following correctly shows the change of $\mathrm{pH}$ of the titraction mixture in this experiment?
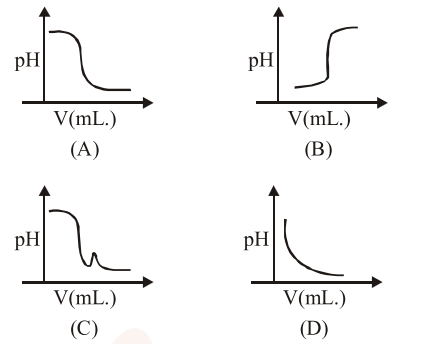
Correct Option: 1
Solution: