Question:
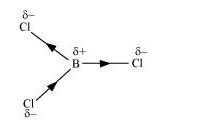
If $\mathrm{B}-\mathrm{Cl}$ bond has a dipole moment, explain why $\mathrm{BCl}_{3}$ molecule has zero dipole moment.
Solution:
As a result of the difference in the electronegativities of B and Cl, the B–Cl bond is polar in nature. However, the BCl3 molecule is non-polar. This is because BCl3 is trigonal planar in shape. It is a symmetrical molecule. Hence, the respective dipole-moments of the B–Cl bond cancel each other, thereby causing a zero-dipole moment.