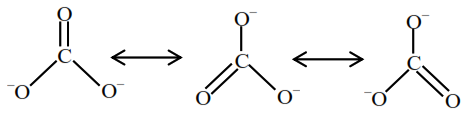
Question:
Identify the species having one $\pi$-bond and maximum number of canonical forms from the following:
Correct Option: , 4
Solution:
Among $\mathrm{SO}_{3}, \mathrm{O}_{2}, \mathrm{SO}_{2}$ and $\mathrm{CO}_{3}^{2-}$, only $\mathrm{O}_{2}$ and $\mathrm{CO}_{3}^{2-}$ has only one $\pi$-bond