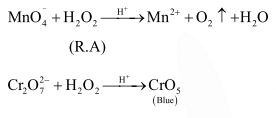
Question:
Hydrogen peroxide acts both as an oxidising and as a reducing agent depending upon the nature of the reacting species. In which of the following cases $\mathrm{H}_{2} \mathrm{O}_{2}$ acts as a reducing agent in acid medium ?
Correct Option: 1
Solution: