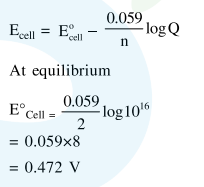
Question:
Given the equilibrium constant :
$\mathrm{KC}$ of the reaction :
$\mathrm{Cu}(\mathrm{s})+2 \mathrm{Ag}^{+}(\mathrm{aq}) \rightarrow \mathrm{Cu}^{2+}(\mathrm{aq})+2 \mathrm{Ag}(\mathrm{s})$ is
$10 \times 10^{15}$, calculate the $E_{\text {cell }}^{0}$ of this reaction at $298 \mathrm{~K}$
$\left[2.303 \frac{\mathrm{RT}}{\mathrm{F}}\right.$ at $\left.298 \mathrm{~K}=0.059 \mathrm{~V}\right]$
Correct Option: , 2
Solution: