Question:
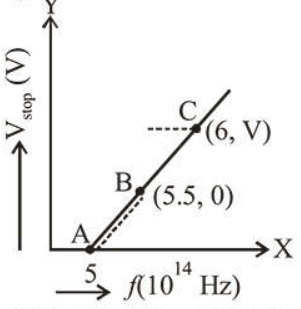
Given figure shows few data points in a photo electric effect experiment for a certain metal. The minimum energy for ejection of electron from its surface is : (Plancks constant $h=6.62 \times 10^{-34} \mathrm{~J} . \mathrm{s}$ )
Correct Option: 1
Solution:
(1) Graph of $V_{s}$ and $f$ given at $B(5.5,0)$
Minimum energy for ejection of electron
$=$ Work function $(\phi)$.
$\phi=h V$ joule or $\phi=\frac{h V}{e} \mathrm{eV}($ for $V=0)$
$\therefore \phi=\frac{6.62 \times 10^{-34} \times 5.5 \times 10^{14}}{1.6 \times 10^{-19}} \mathrm{eV}=2.27 \mathrm{eV}$
