Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R :
Question:
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R :
Assertion A : The H-O-H bond angle in water molecule is $104.5^{\circ}$.
Reason $\mathbf{R}$ : The lone pair - lone pair repulsion of electrons is higher than the bond pair - bond pair repulsion.
Correct Option: 4,
Solution:
$\mathrm{H}_{2} \mathrm{O}$
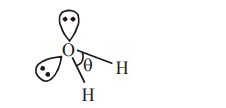
$\theta=104.5^{\circ}$
the hybridisation of oxygen is water molecule is $\mathrm{sp}^{3}$.
So electron geometry of water molecule is tetrahedral and the bond angle should be 109°28" but as we know that lone pair-lone pair repulsion of electrons is higher than the bond pair-bond pair repulsion because lone pair is occupied more space areound central atom than that of bond pair.
