Question:
Given below are two statements :
Statement I : o-Nitrophenol is steam volatile due to intramolecular hydrogen bonding.
Statement II : o-Nitrophenol has high melting due to hydrogen bonding.
In the light of the above statements, choose the most appropriate answer from the options given below:
Correct Option: , 4
Solution:
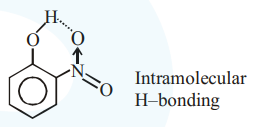
thus it is more volatile due to intramolecular H-bonding.
Melting point depends on packing efficiency not on H-bonding thus statement II is false
