Given below are two statements.
Statement I: In the titration between strong acid and weak base methyl orange is suitable as an indicator.
Statement II: For titration of acetic acid with $\mathrm{NaOH}$ phenolphthalein is not a suitable indicator. In the light of the above statements, choose the most appropriate answer from the options given below:
Correct Option: , 2
Titration curve for strong acid and weak base initially a buffer of weak base and conjugate acid is :
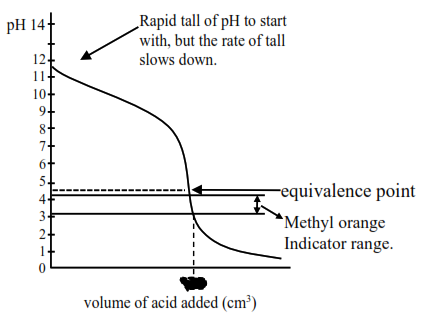
Formed, thus $\mathrm{pH}$ falls slowly and after equivalence point, so the $\mathrm{pH}$ falls sharply so methyl arrange, having $\mathrm{pH}$ range of $3.2$ to $4.4$ will weak as indicator. So statement-I is correct.
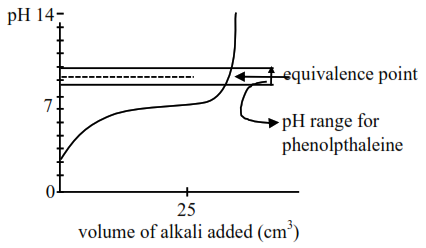
Titration curve for weak acid and strong base (NaOH)
Initially weak acid will form a buffer so $\mathrm{pH}$ increases slowly but after equivalence point. it rises sharply covering range of phenolphthalein so it will be suitable indicator so statement-II is false.
