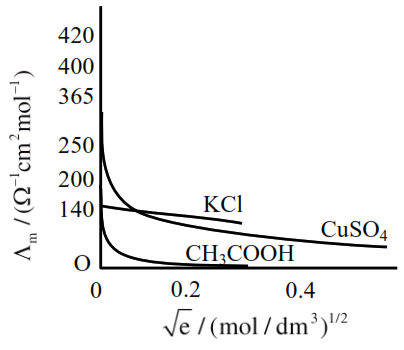
Given below are two statements :
Statement I : The limiting molar conductivity of $\mathrm{KCl}$ (strong electrolyte) is higher compared to that of $\mathrm{CH}_{3} \mathrm{COOH}$ (weak electrolyte).
Statement II : Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below:
Correct Option: , 4
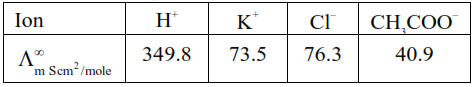
So $\Lambda_{\mathrm{m} \mathrm{CH}_{3} \mathrm{COOH}}^{\infty}=\Lambda_{\mathrm{m}\left(\mathrm{H}^{+}\right)}^{\infty}+\Lambda_{\mathrm{m} \mathrm{CH}_{3} \mathrm{COO}^{-}}^{\infty}$
$=349.8+40.9$
$=390.7 \mathrm{Scm}^{2} / \mathrm{mole}$
$\Lambda_{\mathrm{m} \mathrm{KCl}}^{\infty}=\Lambda_{\mathrm{m}\left(\mathrm{K}^{+}\right)}^{\infty}+\Lambda_{\mathrm{m}\left(\mathrm{Cl}^{-}\right)}^{\infty}$
$=73.5+76.3$
$=149.3 \mathrm{Scm}^{2} / \mathrm{mole}$
So statement-I is wrong or False.
As the concentration decreases, the dilution increases which increases the degree of dissociation, thus increasing the no. of ions, which increases the molar conductance.
So statement-II is false.