Question:
Gaseous cyclobutene isomerizes to butadiene in a first order process which has a 'k' value of $3.3 \times 10^{-4} \mathrm{~s}^{-1}$ at $153^{\circ} \mathrm{C}$. The time in minutes it takes for the isomerization to proceed $40 \%$ to completion at this temperature is _____. (Rounded off to the nearest integer)
Solution:
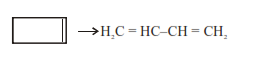
$\mathrm{Kt}=\ln \frac{[\mathrm{A}]_{0}}{[\mathrm{~A}]_{\mathrm{t}}}$
$3.3 \times 10^{-4} \times \mathrm{t}=\ell \mathrm{n}\left(\frac{100}{60}\right)$
$\mathrm{t}=1547.956 \mathrm{sec}$
$\mathrm{t}=25.799 \mathrm{~min}$
26 min
