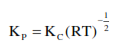Question:
For the reaction $\mathrm{SO}_{2(\mathrm{~g})}+\frac{1}{2} \mathrm{O}_{2(\mathrm{~g})} \rightleftharpoons \mathrm{SO}_{3(\mathrm{~g})}$, if $\mathrm{K}_{\mathrm{p}}=\mathrm{K}_{\mathrm{C}}(\mathrm{RT})^{\mathrm{x}}$ where the symbols have usual meaning then the value of $x$ is :(assuming ideality)
Correct Option: , 4
Solution: