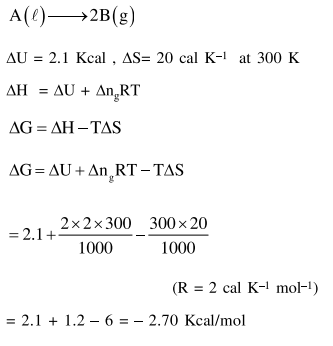
Question: For the reaction ;
$\mathrm{A}(l) \rightarrow 2 \mathrm{~B}(\mathrm{~g})$
$\Delta \mathrm{U}=2.1 \mathrm{kcal}, \Delta \mathrm{S}=20 \mathrm{cal} \mathrm{} \mathrm{K}^{-1}$ at $300 \mathrm{~K}$
Hence $\Delta \mathrm{G}$ in kcal is___________
Solution: