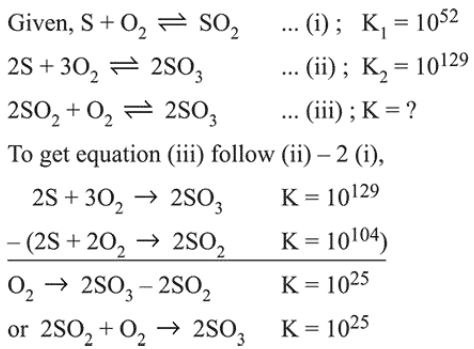
Question:
For the following reactions, equilibrium constants are given :
$\mathrm{S}(\mathrm{s})+\mathrm{O}_{2}(\mathrm{~g}) \rightleftharpoons \mathrm{SO}_{2}(\mathrm{~g}) ; \mathrm{K}_{1}=10^{52}$
$2 \mathrm{~S}(\mathrm{~s})+3 \mathrm{O}_{2}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{SO}_{3}(\mathrm{~g}) ; \mathrm{K}_{2}=10^{129}$
The equilibrium constant for the reaction,
$2 \mathrm{SO}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{SO}_{3}(\mathrm{~g})$ is :
Correct Option: , 3
Solution: