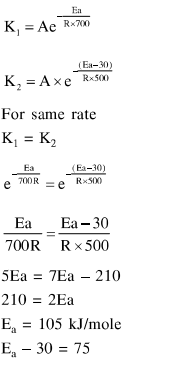
Question:
For the following reactions
$\mathrm{A} \stackrel{700 \mathrm{~K}}{\longrightarrow}$ Product
$\mathrm{A} \underset{\text { catalyst }}{\stackrel{500 \mathrm{~K}}{\longrightarrow}}$ Product
it was found that $E_{a}$ is decreased by $30 \mathrm{~kJ} / \mathrm{mol}$ in the presence of catalyst.
If the rate remains unchanged, the activation energy for catalysed reaction is (Assume pre exponential factor is same):
Correct Option: , 4
Solution: