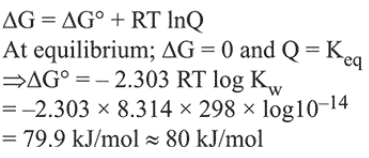Question: For the equilibrium$2 \mathrm{H}_{2} \mathrm{O} \rightleftharpoons \mathrm{H}_{3} \mathrm{O}^{+}+\mathrm{OH}^{-} ;$the value of $\Delta \mathrm{G}^{\circ}$ at $298 \mathrm{~K}$ is approximately:
$100 \mathrm{~kJ} \mathrm{~mol}^{-1}$
$-80 \mathrm{~kJ} \mathrm{~mol}^{-1}$
$80 \mathrm{~kJ} \mathrm{~mol}^{-1}$
$-100 \mathrm{~kJ} \mathrm{~mol}^{-1}$
Correct Option: , 3
Solution: