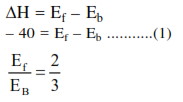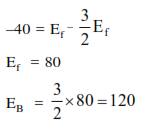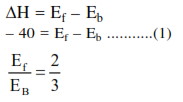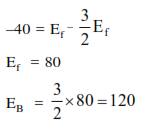Question: For the equilibrium, $\mathrm{A}(\mathrm{g}) \rightleftharpoons \mathrm{B}(\mathrm{g}), \Delta \mathrm{H}$ is $-40 \mathrm{~kJ} / \mathrm{mol}$. If the ratio of the activation energies of the forward $\left(E_{f}\right)$ and reverse $\left(E_{b}\right)$ reactions is $\frac{2}{3}$ then :-
$\mathrm{E}_{\mathrm{f}}=60 \mathrm{~kJ} / \mathrm{mol} ; \mathrm{E}_{\mathrm{b}}=100 \mathrm{~kJ} / \mathrm{mol}$
$\mathrm{E}_{\mathrm{f}}=30 \mathrm{~kJ} / \mathrm{mol} ; \mathrm{E}_{\mathrm{b}}=70 \mathrm{~kJ} / \mathrm{mol}$
$\mathrm{E}_{\mathrm{f}}=80 \mathrm{~kJ} / \mathrm{mol} ; \mathrm{E}_{\mathrm{b}}=120 \mathrm{~kJ} / \mathrm{mol}$
$\mathrm{E}_{\mathrm{f}}=70 \mathrm{~kJ} / \mathrm{mol} ; \mathrm{E}_{\mathrm{b}}=30 \mathrm{~kJ} / \mathrm{mol}$
Correct Option: , 3
Solution: