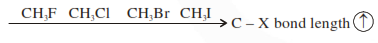Question: For the compounds $\mathrm{CH}_{3} \mathrm{Cl}, \mathrm{CH}_{3} \mathrm{Br}, \mathrm{CH}_{3} \mathrm{I}$ and $\mathrm{CH}_{3} \mathrm{~F}$, the correct order of increasing $\mathrm{C}$-halogen bond length is :
$\mathrm{CH}_{3} \mathrm{~F}<\mathrm{CH}_{3} \mathrm{Br}<\mathrm{CH}_{3} \mathrm{Cl}<\mathrm{CH}_{3} \mathrm{I}$
$\mathrm{CH}_{3} \mathrm{~F}<\mathrm{CH}_{3} \mathrm{Cl}<\mathrm{CH}_{3} \mathrm{Br}<\mathrm{CH}_{3} \mathrm{I}$
$\mathrm{CH}_{3} \mathrm{Cl}<\mathrm{CH}_{3} \mathrm{Br}<\mathrm{CH}_{3} \mathrm{~F}<\mathrm{CH}_{3} \mathrm{I}$
$\mathrm{CH}_{3} \mathrm{~F}<\mathrm{CH}_{3} \mathrm{I}<\mathrm{CH}_{3} \mathrm{Br}<\mathrm{CH}_{3} \mathrm{Cl}$
Correct Option: , 2
Solution: