Question:
For a reaction, $4 \mathrm{M}(\mathrm{s})+\mathrm{nO}_{2}(\mathrm{~g}) \rightarrow 2 \mathrm{M}_{2} \mathrm{O}_{\mathrm{n}}(\mathrm{s})$, the free energy change is plotted as a function of temperature. The temperature below which the oxide is stable could be inferred from the plot as the point at which :
Correct Option: , 2
Solution:
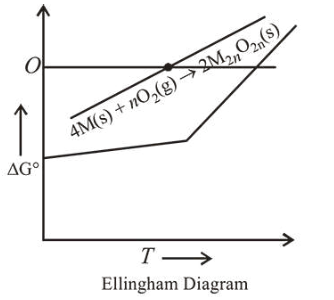
From graph it is evident that the temperature below which the oxide is stable, is the point at which free energy change shows a change from negative to positive.
