Question:
For a certain radioactive process the graph between In $\mathrm{R}$ and $\mathrm{t}(\mathrm{sec})$ is obtained as shown in the figure. Then the value of half life for the unknown radioactive material is approximately:
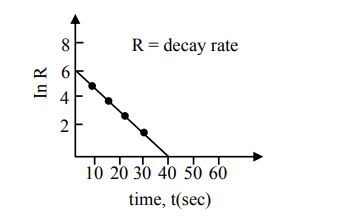
Correct Option: , 4
Solution:
$\mathrm{R}=\mathrm{R}_{0} \mathrm{e}^{-\lambda \mathrm{t}}$
$\ell \mathrm{nR}=\ell \mathrm{nR}_{0}-\lambda \mathrm{t}$
$-\lambda$ is slope of straight line
$\lambda=\frac{3}{20}$
$\mathrm{t}_{1 / 2}=\frac{\ell \ln 2}{\lambda}=4.62$
