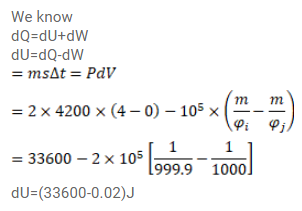Question:
Find the change in the internal energy of $2 \mathrm{~kg}$ of water as it is heated from $0^{\circ} \mathrm{C}$ to $4^{\circ} \mathrm{C}$. The specific heat capacity of water is $4200 \mathrm{~J} / \mathrm{kg}-\mathrm{K}$ and its densities at $0^{\circ} \mathrm{C}$ to $4^{\circ} \mathrm{C}$ are $999.9 \mathrm{~kg} / \mathrm{m}^{3}$ and $1000 \mathrm{~kg} / \mathrm{m}^{3}$ respectively. Atmospheric pressure $=10^{6} \mathrm{~Pa}$.
Solution: