Question:
Find out the valency of the atoms represented by the Fig. (a) and (b).
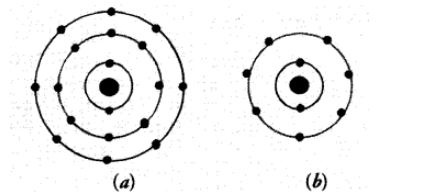
Solution:
(a) The electronic configuration of the atom is $2,8,8$. It has completely filled $K, L, M$ shells. Its valency is zero. The atom belongs to the element Argon (Ar).
(b) The electronic configuration of the atom is 2,7 . It has seven electrons in the valence shell. Its valency is $(8-7)$ equal to one. The atom belongs to the element fluorine (F).
