Question:
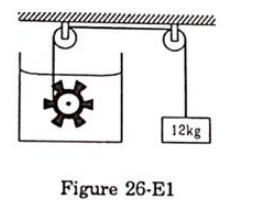
Figure (26.E1) shows a paddle wheel coupled to a mass of $12 \mathrm{~kg}$ through fixed frictionless pulleys. The paddle is immersed in a liquid of heat capacity $4200 \mathrm{~J} / \mathrm{K}$ kept in an adiabatic container. Consider a time interval in which the $12 \mathrm{~kg}$ block falls slowly through $70 \mathrm{~cm}$.
(a) How much heat is given to the liquid?
(b) How much work is done on the liquid?
(c) Calculate the rise in the temperature of the liquid neglecting the heat capacity of the container and the paddle.
Solution:

