Question:
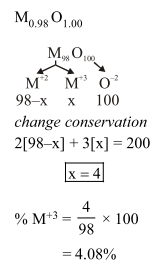
Expermentally it was found that a metal oxide has formula $\mathrm{M}_{0.98} \mathrm{O}$. Metal $\mathrm{M}$, is present as $\mathrm{M}^{2+}$ and $\mathrm{M}^{3+}$ in its oxide. Fraction of the metal which exists as $\mathrm{M}^{3+}$ would be :-
Correct Option: , 2
Solution: