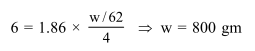Question:
Ethylene glycol is used as an antifreeze in a cold climate. Mass of ethylene glycol which should be added to $4 \mathrm{~kg}$ of water to prevent it from freezing at $-6^{\circ} \mathrm{C}$ will be :
$\left(\mathrm{K}_{\mathrm{f}}\right.$ for water $=1.86 \mathrm{~K} \mathrm{kgmol}^{-1}$, and molar mass of ethylene glycol $\left.=62 \mathrm{gmol}^{-1}\right)$
Correct Option: , 3
Solution: